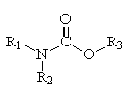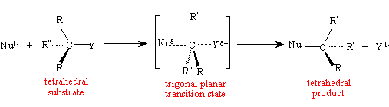| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z |
|
Acetylcholine is a chemical in the body that functions as a neurotransmitter. It sends electrical impulses across junctions between nerve cells, and from motor neurons to muscle cells, causing the muscle cells to contract. As an electric impulse reaches the nerve ending, the nerve cell releases acetylcholine, which passes across the junction and bonds chemically with a receptor molecule in the membrane of the neighboring nerve cell. The bonding of acetylcholine to the receptor molecule alters the polarity and permeability of the membrane so that the nerve impulse can be transmitted. The effect of acetylcholine can be neutralized by an enzyme, such as cholinesterase, which decomposes acetylcholine through the process of hydrolysis. When acetylcholine is decomposed, the muscle relaxes. Abiotic transformation is the degradation process of organic chemicals via chemical (hydrolysis) or physical (photolysis, volatilization) reactions. Bioaccumulation of a substance is its capacity to accumulate in the tissues of organisms either through direct exposure to water, air or soil or through consumption of food. It is calculated as the ratio, in a steady-state situation, of its concentration in the organism to the concentration in the medium to which this organism is exposed (Bioaccumulation Factor, BAF ). When the intake in the organism is only due to the substance dissolved in the medium, generally water, the ratio is called the Bioconcentration Factor (BCF). Generally, fish are the preferred test organisms. As the tendency of organic substances to bioconcentrate in tissue has often been related to their hydrophobicity or lipophilicity, it is suggested, when BAF or BCF values are not available, the logarithm of the substance's octanol-water partition coefficient ( log Kow) be used to estimate the bioconcentration potential. The use of this coefficient does not consider the metabolism and implies biological stability, i.e. the absence of metabolic pathways for biodegradation.Consequently, criteria recommended for bioaccumulation are preferably based on the BAF or BCF values. If they are not available, the log Kow, used with scientific judgment, may be a useful screening criterion. To be considered as liable to bioaccumulate through the food chain a substance must be characterized by either a BAF or BCF value higher than 5000 , or in the absence of available BAF or BCF data, an octanol-water partition coefficient, log Kow, higher than 5.0. Remarks: Substances which have molecular weights higher than 600, or which are characterized by a log Kow higher than 7, typically have molecular structures too large to cross biological membranes and bioaccumulate. In these cases, log Kow data must be interpreted very cautiously. Biodegradation is the complete biochemical decomposition of organic molecules by microorganisms.Biodegradability reduces a substance being emitted into the environment or a substance being retained by it. The more complete the biodegradation of a product within a given time, the less important other ecological protection aspects become. Generally, materials are considered biodegradable if they degrade in the particular test system after 28 days by more than 70 %. Bioconcentration is the increase in concentration of a chemical in an organism resulting from tissue absorption levels exceeding the rates of metabolism and excretion. Biodegradation is the complete biochemical decomposition of organic molecules by microorganisms.Biodegradability reduces a substance being emitted into the environment or a substance being retained by it. The more complete the biodegradation of a product within a given time, the less important other ecological protection aspects become. Generally, materials are considered biodegradable if they degrade in the particular test system after 28 days by more than 70 %. Biological degradation is the biochemical transformation of organic molecules by microorganisms. Biological degradation may be complete, that is inorganic products such as CO2 and H2O are the final products or incomplete. Incomplete biological degradation can lead to products that are of equal or more concern than the parent compound. Generally, materials are considered biodegradable if they degrade in the particular test system after 28 days by more than 70 %. Biotic transformation is the degradation process of organic chemicals via metabolism of microorganisms. Carbamates are a group of pesticides derived from carbamic acid (HO-CO-NH2).
The R1 and R2 are hydrogens or carbon-centered groups and R3 is a carbon-centered group. The carbamates were introduced in the early 1950s by the Swiss company, Geigy Chemical Company. They have the same mode of action as organophosphate pesticides, which is inhibition of an enzyme, cholinesterase, vital to the nervous system. Carbaryl and carbofuran are carbamate pesticides. Carbaryl or 1-naphthyl methylcarbamate is a carbamate insecticide, which was initially available in the mid-1950s. It is the most widely used carbamate. It is sold under the tradename of Sevin and is used in the lawn and garden area to control insects. It has an oral LD50 of 307 and a dermal LD50 of 2,000 (Ware, 1978). For more information see: http://www.speclab.com/compound/c63252.htm http://pmep.cce.cornell.edu/profiles/extoxnet/carbaryl-dicrotophos/carbaryl-ext.html Carbocation is a hydrocarbon, which contains a central carbon atom with 3 other groups attached and bearing only 6 electrons. This results in a positively charged species. Carbofuran or 2,3-dihydro-2,2-dimethyl-7-benzofuranyl methylcarbamate is a carbamate insecticide. It is also used as a nematicide, which is used to kill microscopic roundworms that attack the roots of crops. Carbofuran is not licensed for home use. It has an oral LD50 of 8 and a dermal LD50 of 10,200 (Ware, 1978). Choline is a natural amine, often classed in the vitamin B complex and a constituent of many other biologically important molecules, such as acetylcholine and lecithin. It's a natural substance from foods, and needed by the body to make acetylcholine. Cholinesterase is an enzyme found at nerve terminals that inactivates acetylcholine by hydrolyzing it to form acetic acid and choline. Certain pesticides act as cholinesterase inhibitors and prevent the formation of the enzyme. Without cholinesterase, acetylcholine is not inactivated and the nerve keep firing or transmitting electrical pulses without relief. Daphnia magna, Daphnia pulex, and Ceriodaphnia dubia are freshwater microcrustaceans, commonly refered to as water fleas, belonging to the class of Crustacea.The selection of Daphias for routine use in toxicity tests is appropriate for a number of reasons: -They are broadly distributed in freshwater bodies and are present throughout a wide range of habitats. -They are important links in many important food chains and a significant source of food for small fish. -They have a relative short life cycle and are relatively easy to culture in the laboratory. -They are sensitive to a broad range of aquatic contaminants and widely used as test organisms for evaluating acute and chronic toxicity of chemicals, and, -Their small sizes require only small volumes of test and dilution water. The reasons for selecting fathead minnow and rainbow trout for routine toxicity tests are: -Extensive toxicological database -Proven sensitivity to aquatic toxicants -Widespread availability. Direct photolysis is a photochemical reaction in which a molecule absorbs the light energy necessary to cause a transformation to the molecule. Compare to indirect photolysis. EPA is the United State Environmental Protection Agency. Electrophiles are electrons deficient species that seek out electron rich areas and are capable of accepting a pair of electrons to form a new covalent bond. Endocrine-disruptor is a chemical that mimics or inhibits the effects of hormones. Endosulfan sulfate is an oxidation by-product of endosulfan mediated by microorganism.It seems to be the dominant product detected in the soil and/or sediment samples. An excited state is a higher energy level (unstable) where a bond-breaking or bond-making process has more probability of occurring. GC-ECD is a gas chromatograph with an electron capture detector. Gas chromatography (GC) is a separation technique that uses gas as a mobile phase and a liquid or solid as a stationary phase coated on a column. Organic compounds are separated because of their different interactions with the stationary phase. Different devices are available for the detection of the compounds as they exit the column. ECD is an electron capture detector. FID is a flame ionization detector. A ground state is the lowest-energy (most stable) arrangement of electrons. HPLC (high-performance liquid chromatography) is an analytical technique for mixtures that uses liquid as a mobile phase and use liquid or solid as a stationary phase. Analytes are separated based on their affinity for the column that serves as the stationary phase. The essential components include a pump or pumps, sampling valves and loops, a separation column, a detector, and a readout device.Various detectors can be used in conjunction with the HPLC including UV-vis spectrometer, fluorescence detectors, and electrochemical detectors. Half-life is the time required for 50 percent of the compound to disappear. Henry’s Law constant - the solubility of a gas in a liquid is proportional to the pressure of the gas over the solution.The following formula can be applied for Henry’s Law 1.CaP 2.C=kP Where c is the molar concentration (mol/L) of the dissolved gas and P is the pressure (in atm) of the gas over the solution. k for a given gas is the Henry's Law constant dependent only on temperature. Hydrolysis is a bond breaking and bond forming reaction in which a molecule R-X, where X is a leaving group, reacts with water (H2O) or hydroxide ion (OH-) to form a new R-O bond and cleave a R-X bond in the original molecule. The products of hydrolysis reactions are usually less of an environmental concern than the parent compounds because they are usually more polar compounds which are less hydrophobic than the original molecules and therefore their behavior in the environment is less problematic. To learn more about hydrolysis, see the case studies on endosulfan and carbaryl. Hydrophobic compound is an organic molecule that has a log Kow greater than 2 (log Kow>2). Indirect photolysis is a photochemical reaction in which an intermediate known as a photosensitizer is energized and then transfers energy to the organic contaminant. Compare to direct photolysis. Infrared energy is electromagnetic radiation with wavelengths greater than 750 nm which is low in energy compared to ultraviolet radiation. Irradiance is the radiant flux of all incident light on an infinitesimal element of surface containing the point of interest divided by the area of that element. The SI unit is the watt per square meter. LC50 or lethal concentration is the concentration of a toxic substance in the environment [water or air] that causes the death of 50% of the exposed group of organisms within a specified period of time. The duration of the exposure time should be indicated (eg. 7-d LC50 = LC50 after an exposure time of 7 days). LC50 is usually reported as parts per million [ppm] - volume/volume or weight/volume or as cubic centimeters [cm3] or milligrams [mg] per cubic meter [m3]. 96 hr LC50 is the concentration at which 50 percent of the test organisms die within 96 hours of exposure Liquid- Liquid Extraction is a separation process that takes advantage of the relative solubilities of solutes in immiscible solvents. The solute dissolves more readily and becomes more concentrated in the solvent in which it has a higher solubility. A partial separation occurs when a number of solutes have different relative solubilities in the two solvents used. Millieinstein is one millimole of photons. The einstein is the equivalent of 1 mole of photons or 6.02 x 1023 photons. The einstein is not sanctioned by the IUPAC (International Union of Pure and Applied Chemistry).Molar absorbance coefficient is related to the absorption of electromagnetic radiation by a substance. It indicates the probability of an electronic transition in a chromophore. The molar absorbance coefficient is the absorbance divided by the absorption pathlength and the concentration. The SI units are square meters per mole (m2 mol-1). Also known as the molar extinction coefficient.Nucleophiles are electron rich species that seek out electron deficient areas and are capable of donating a pair of electrons to form a new covalent bond. Octanol/water partition coefficient (Kow) is the ratio of a chemicals concentration in the octanol phase to its concentration in the aqueous phase of a two-phase octanol/water at equilibrium system. Chemists use the Kow of a compound as an indication of its hydrophobicity. The Kow can be expressed as below: Kow = concentration in octanol phase = Co concentration in aqueous phase Cw The values of Kow are unitless. The concept was developed by chemists studying the partitioning of pharmaceuticals in the body. Octanol serves as a good model or surrogate for fatty tissue. An organic carbon partition coefficient (Koc) is the ratio of a chemicals concentration in the organic carbon phase to its concentration in the aqueous phase at equilibrium. The organic carbon phase represents the natural organic matter that is found in sediments and soils. There is a positive correlation between the Kow of a compound and its Koc. The Koc can be expressed as below:Koc = concentration in organic carbon = Coc(mol/kg) concentration in aqueous phase Cw (mol/L) Organic Sulfite is an organic molecule containing a S atom with an oxidation state equal one. Organochlorine pesticides are some of the first organic pesticides that were developed after World War II. The organochlorine pesticides do not belong to a single class of chemical compounds but range from chlorinated aliphatic hydrocarbons to cyclodienes. Many of the organochlorine pesticides have been banned in the U.S. such as DDT, endrin, dieldrin, and chlordane. However, DDT is still used in many tropical areas because of its effectiveness against malaria bearing insects. Organophosphate pesticides are pesticides derived from phosphoric acid esters. They are mainly used as insecticides although a few are used as herbicides and fungicides. Common organophosphate pesticides include malathion, methyl parathion, parathion, phorate, terbufos, and disulfoton. Organophosphate pesticides are subject to hydrolysis which decreases their persistence in the environment. They are generally more toxic to vertebrates than organochlorine insecticides An oxidation-reduction reaction is a reaction in which electrons are transferred between two reactants. Whenever a substance is oxidized, another substance must be reduced. Similarly, when a substance is reduced, another substance must be oxidized. The substance that is oxidized is called the reducing agent. The substance that is reduced is called the oxidizing agent. An oxidation is defined as the loss of an electron or electrons and reduction is the gain of an electron or electrons. A simple scheme of an oxidation-reduction reaction is shown below.
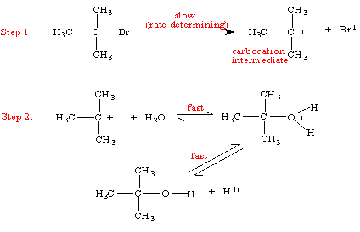
Further information about oxidation-reduction reactions can be found at: http://chem.boisestate.edu/~rcbanks/inorganic/ Pesticide is a substance or material used to kill any undesirable or unwanted fungi, plants, insects, or any organisms.This generic term is used to describe fungicides, algicides, herbicides, insecticides, rodenticides, and other substances pH is a measure of acid or base, as determined by negative log of the hydrogen ion concentration. A photochemical reaction is a chemical reaction initiated by absorption of light. Photosensitizer is a light-absorbing molecule that mediates an indirect photolysis reaction. Plasticizer is a chemical agent that is added to a polymer to make it softer and more flexible. These are usually small molecules with dangling bits that can disrupt the packing of polymer chains. A common plasticizer used to soften polyvinly chloride (PVC) is dioctyl phthalate. Pseudo first order is a reaction in which the concentrations of all but one of the reactants are so large that they change a little over the course of the reaction; or in other words, these concentrations are constant at a given system. For example, the rate of cometabolic biotransformation of some halogenated organic compounds or biological transformation can be expressed as: -dC/dt=kCX whereC is the concentration of organic compound transformed X is the concentration of bacteria Minus sign (-) represents the disappearance of organic compounds During biotransformation process, X might be so large that would not affect the reaction; subsequently, X can be considered as a constant and the rate of statement becomes: -dC/dt=k'C where k' is equal to kX and the reaction in this form is typically termed a pseudo-first-order reaction with k' being the pseudo-first-order rate constant. Rate-determining step: The rate-determining step is the slowest step in a multi-step reaction. It determines the overall rate constant for the reaction because it has the transition state with the highest free energy. SN1 (unimolecular nucleophilic substitution) is a mechanism that occurs in at least two distinct steps. The first step is that the leaving group leaves with the formation of a carbocation. The second step is that the nucleophile donates electrons to neutralize the carbocation and make a new bond to carbon. The SN1 mechanism depends on the formation of a carbocation. The general trends of SN1 mechanism are: A). Leaving groups located in a primary position NEVER undergo SN1 reactions B). Leaving groups located in a secondary position can undergo SN1 reactions, but it depends on the structure and other factors. C). Leaving groups located in a tertiary position ALWAYS undergo SN1 reactions, compared to SN2 reactions The mechanism of SN1 reaction
The major factors affecting the rates of SN1 reactions are substrate, nucleophile, leaving group, and solvent system SN2 (biomolecular nucleophilic substitution) is a mechanism where a substitution takes place all in one step. The leaving group departs and the nucleophile attaches at the same time. The SN2 reaction occurs when the nucleophile can easily approach the carbon; however, it fails to occur when there is a lot of crowding around that carbon. The SN2 reaction is very sensitive to steric hindrance. The general trends of SN2 mechanism are A). Leaving groups located in a primary position easily undergo SN2 reactions. B). Leaving groups located in a secondary position can undergo SN2 reactions, but it depends on the structure and other factors. C). Leaving groups located in a tertiary position do NOT undergo SN2 reactions. The mechanism of SN2 mechanism:
The major factors affecting the rates of SN2 reactions include substrate, nucleophile, leaving group and solvent system. Toxicant is an agent that can produce an adverse effect in a biological system, seriously damaging its structure or function or producing death. Ultraviolet light is electromagnetic radiation with wavelengths between 50 and 400 nm. which is high in energy content compared to infrared radiation. An ultraviolet/visible spectrophotometer is an analytical instrument that is designed to measure the amount of light absorbed at wavelengths characteristic of ultraviolet and visible region. Further information about UV/visible spectroscopy can be found at http://www.onu.edu/A+S/chemistry/ultra.html and http://www.imp.mtu.edu/matchar/uv.html. Visible light is electromagnetic with wavelengths between 400(violet light) to 750 (red light) nm which is of intermediate energy. |