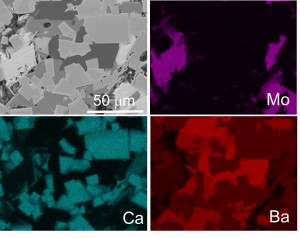
Ceramic Waste Forms for Nuclear Energy: Durable ceramic waste forms that incorporate a wide range of radionuclides have the potential to broaden the available disposal options and to lower the storage and disposal costs associated with advanced fuel cycles. Assemblages of several titanate phases have been successfully demonstrated to incorporate radioactive waste elements, and the multiphase nature of these materials allows them to accommodate variation in the waste composition. A major technical hurdle to the use of multiphase oxide waste forms is the ability to characterize the complex elemental partitioning that occurs, particularly at the grain boundary interface in crystalline systems, as well as to predict the long-term performance of these material systems.
For more information, see our paper in Chemistry of Materials and Journal of Alloys and Compounds.
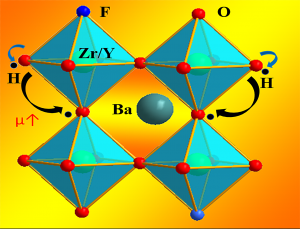
Solid State Ionics for Energy Conversion and Storage: Ceramic membranes which transport ions play an essential role in a number of energy conversion systems including solid oxide fuel cells (SOFC), Li-air batteries, oxygen separation and permeation membranes including fuels synthesis. In addition to energy generation systems, ceramic membranes have the potential to be increasingly prominent in advanced manufacturing processes including electrochemical reduction cells for metals processing and spent nuclear fuel re-processing as well as high temperature state of the art combustion control sensors. The properties and function of these devices are controlled by mixed ionic and electronic conductivity (MIEC) in the respective material system. Dr. Brinkman’s group address fundamental aspects of MIEC materials including the characterization of surface and interfacial properties that impact performance. Work on SOFCs focuses on protonic conducting oxides with improved stability to CO2 and H2O exposure ii) understanding and controlling the performance of grain boundaries in these systems that often exhibit a “blocking effect” limiting performance and iii) decreasing the operating temperature through the use of tailored interfaces.
For more information, see our paper in Nature Communications, (https://www.nature.com/articles/ncomms7824?origin=ppub), Journal of Materials Science (https://link.springer.com/article/10.1007/s10853-019-03559-9), and Journal of Alloys and Compounds (https://www.sciencedirect.com/science/article/pii/S0925838816337586)

Experimental Thermodynamics : Melt solution calorimetry and vapor phase adsorption calorimetry are unique techniques used to advance our fundamental understanding of a wide range of materials used in energy conversion and storage applications including nuclear energy. In the wide range of applications studied by the Brinkman group, the functionality of materials is provided by dopant additions that impact the structure and stability. Thermodynamic measurements such as calorimetry, provide a direct measure of the energetics of defect formation reactions as well as the formation energies of the resulting structures. This information is essential for the rational design of materials and prediction of their long-term performance and stability. High temperature oxide melt solution calorimetry is a versatile technique for studying the energetics of formation, solid solution mixing, phase transition, and order/disorder in ceramics making it a general tool with diverse applications in geochemistry, mineralogy, materials science, ceramics, and solid-state chemistry.
For more information, see our paper in Journal of Materials Science (https://link.springer.com/article/10.1007/s10853-018-2904-1) and the Journal of the American Chemical Society (https://pubs.acs.org/doi/abs/10.1021/jacs.9b04737)
