|
|
Case Study in Environmental Chemistry....
Case Study 4: Kinetics of Carbaryl Hydrolysis
Author: Sarunya Hengpraprom and Cindy M.Lee, Environmental Engineering and Science, Clemson University.
For further detail choose the appropriate section
Hydrolysis kinetics
The rate law for hydrolysis is usually defined by a simple pseudo-first order reaction.
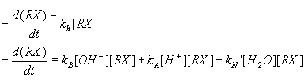
where [RX] is the molar concentration of the chemical,
kh is the observed pseudo-first order rate constant for hydrolysis at a given pH,
kB is the alkaline second order rate constant,
kA is the acid-catalyzed second order rate constant, and
kN' is the neutral second order constant.
By taking into account the acid-catalyzed, neutral, and alkaline hydrolysis reaction, we can express the observed pseudo-first order reaction as:
![]() (4)
(4)
and since [H2O] generally remains constant, we can simplify equation 4 to;
![]() (5)
(5)
In this equation, kA, and kB are the second-order reaction rate constants for acid-catalyzed, alkaline-hydrolysis, respectively, and kN is the first order rate constant for neutral hydrolysis (the alkaline and acid-catalyzed rate constants have the dimensions of M-1s-1 and the neutral rate constant has the dimensions of s-1) (11).
At equilibrium,
Kw = [H+ ] [OH-] (6)
thus, [OH-] = Kw/[H+] (7)
Substituting equation 7 into equation 5 yields,
![]() (8)
(8)
Equation 8 shows how the pH affects the overall rate of hydrolysis. At high or low pH (high OH- or H+) one of the first two terms is usually dominant, while at pH 7 the last term may be the most important. However, the detailed relationship of pH and rate constant depends on the specific values of kA, kB, and kN. Each separate rate constant indicates a stoichiometric relationship between the compounds to be hydrolyzed and the reactants (acid, base, or water), but it does not indicate the detailed mechanism or pathway, which may change from one class to another (11).
As can be seen from equation 8, the hydrolysis reaction is strongly dependent on the pH. A slight change in pH causes a large change in the rate of reaction and/or the hydrolysis half-life. When a reaction follows first-order kinetics, the concentration decreases exponentially with time. According to equation 2, a plot of the natural log of concentration (ln[RX]t) versus time will vary linearly with a slope of -kh. This slope and the rate are dependent on the concentration. The hydrolysis half-life, the time required for 50% of the compound to disappear, will be determined for first-order and pseudo-first order reactions by (12):
![]() (9)
(9)
The larger the overall rate of reaction, the smaller the half-life will be obtained.


