(S. M. Bower)
Isopropyl Alcohol MOVIE
(S. M. Bower)
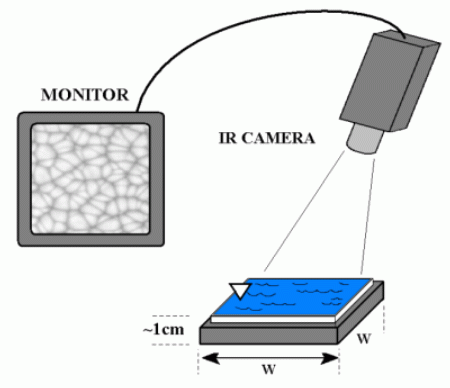
Our research group uses infrared (IR) imaging of liquid surfaces (usually water) for several things including the study of evaporation, natural convection, forced convection and mixed convection. To get started, let's look at a sample movie showing isopropyl alcohol being poured into a small tank. A schematic showing the position of the IR camera with respect to the tank is also shown. In this movie and those that follow, the field of view ranges from 2 inches to 5 inches on a side, and the gray scale is such that warm fluid is white and cold fluid is black.
|
|
|
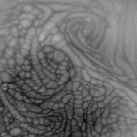
At the end of the above movie, you can see cellular structures forming. These are due to natural convection in the liquid. Note that while the liquids imaged above here are all transparent to the naked eye, they are opaque in the IR...thus, what we are seeing here are images of the liquid surface.
The following are movies, similar to the one above, but for different liquids. Also, the movies begin after the liquid has been poured into the tank. So, the development of the natural convection structures shown in the previous movie, has already occurred.
|
|
|
|
|
The liquids above labeled 'FC' are a special class of liquids called perfluorinated hydrocarbons. These were obtained courtesy of the 3M company.
In some of the movies above, the outline of a finger can be seen. Note that as the finger touches the liquid surface, skin oils change the appearance of the water surface. This is primarily due to the presence of a surfactant monolayer. The fluids in the movies above are solvents and monolayers are quickly dissolved into the bulk. Surfactant monolayers are single molecule thick layers that impart an elasticity to an otherwise clean surface, and they form very easily on water surfaces. The presence of a monolayer damps subsurface motion and changes the temperature field on the surface. This can be seen in the two images below, where the image on the left is a temperature field of a clean water surface, while that on the right is for a surface covered with a monolayer of oleyl alcohol. The heat fluxes are identical in both cases. Each image is ~16 cm on a side.
|
|
|
A movie of a water surface with a monolayer on it is presented below. Note how the structures are larger, and the time evolution is slower, than the movies of the solvents presented earlier.
|
|
All of the above movies and images are for the case where the air above the liquid surface is quiescent. We also study what happens when there is a flow of air over the water surface. The following is an IR image of a water surface experiencing a wind of 4 m/s. The wind flow is from bottom to top:
|
|
The elongation of the structures in the direction of the wind is clearly evident in the above image.
A surfactant monolayer can be dispersed by air shear on the water surface. The following video shows a surfactant-covered water surface visualized in the IR. An air jet is impinging on the water in the center of the image. This jet pushes the surfactant monolayer away from the stagnation point of the jet. Upon removal of the air jet, the surfactant monolayer springs back into place.
|
|
Last Updated October 10, 2008.