Research
“The heart has its reasons of which reason knows nothing.”
Blaise Pascal (1623 – 1662)
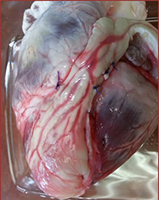
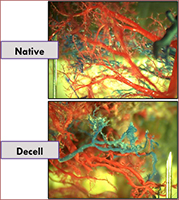
Cardiac Tissue Engineering
According to the American Heart Association, about 735,000 people in the U.S. have heart attacks each year. The cascade of events that follows myocardial infarction include cardiomyocyte death, inflammation, the formation of a non-contractile fibrous scar, alteration in the load of the surrounding myocardium, and finally congestive heart failure. Adult stem cell-based therapies for cardiac repair have great promise as new treatments for heart disease. The goal of this research is to generate a fully vascularized myocardial graft, able to replace the infarct scar tissue. Acellular scaffolds are developed from porcine left-ventricular tissues, seeded with adipose tissue-derived stem cells, and subjected to mechanical, electrical, and biochemical stimuli, reflective of the cardiac environment. The feasibility of infarct scar surgical replacement with the myocardial flap is evaluated in a porcine model.
Collaborator: Dr. Chris Wright
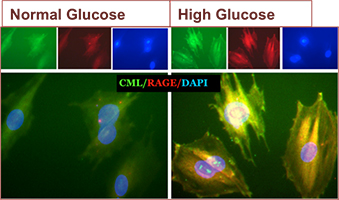
Tissue Engineering Models for Diabetic Cardiomyopathy
Diabetic cardiomyopathy is defined as a ventricular dysfunction that occurs in the absence of hypertension, coronary artery and valve disease. The exact mechanisms are unknown, but there is growing evidence that oxidative stress is a major risk factor for the development of micro-vascular pathogenesis in the myocardium, which results in myocardial cell death, hypertrophy, fibrosis, abnormalities of calcium homeostasis and endothelial dysfunction. The first change noticed in the diabetic heart is altered energy metabolism; in response to a chronic high plasma concentration of long-chain fatty acids and an impaired glucose uptake, glycolysis, and pyruvate oxidation, the heart switches to exclusively using fatty acids for energy supply. The subsequent lipid overload results in an increased production of reactive oxygen species and accumulation of lipid intermediates in cardiomyocytes. Over time these metabolic changes introduce structural changes that affect cardiac contractile characteristics. Our objective is to investigate the early mechanisms that occur in diabetic myocardium in a tissue engineered 3D model.
Collaborator: Dr. John Bruch
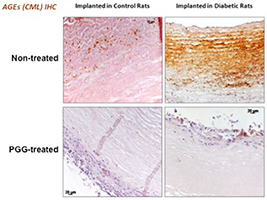
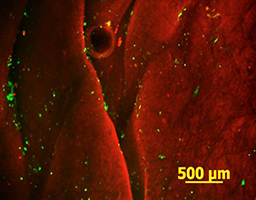
Vascular Tissue Engineering in Diabetes
Diabetes is a major risk factor for vascular diseases and diabetic patients are more prone to undergo surgery for repair or replacement of blood vessels. In diabetes, elevated levels of blood glucose and lipids interact irreversibly with long-lived proteins, such as collagen and elastin from the blood vessel wall, via oxidation and crosslinking processes, resulting in formation of advanced glycation end products (AGEs); the consequence is vascular stiffening, the hallmark of diabetes. Furthermore, cells respond to diabetes-related altered environment by endothelial dysfunction and pathological remodeling that contribute to the onset and progression of vascular disease. Together, these severe cell and extracellular matrix changes result in activation of inflammation, impaired healing, fibrosis, and ectopic calcification, all of which are not conducive to the desired integration of tissue engineered constructs. Our goals are to understand the diabetes-related alterations in implanted tissue engineered blood vessels and to develop vascular grafts resistant to these changes, by treating them with a polyphenol antioxidant and stabilizing agent.
Collaborators: Dr. Gene Langan, Dr. John Bruch
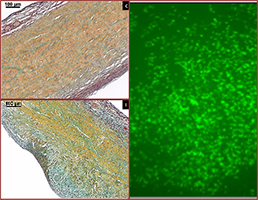
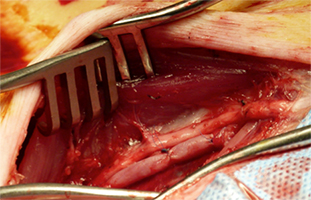
Mitral Valve Tissue Engineering
The human mitral valve apparatus is a large bicuspid, fully supported and fully flexible structure. The large orifice area, the systolic constriction of the posterior left ventricular wall around the mitral annulus, the excess leaflet surface area for coaptation, and the annulus-papillary muscle continuity through chordae are essential features for the correct function of the valve. Degenerative mitral valve disease with elongation or rupture of the chordae apparatus, annular dilatation, and leaflet prolapse, results in valve regurgitation and ventricular dysfunction. In this study, our objective is to develop a tissue-engineered mitral valve, based on an acellular porcine mitral valve scaffold and adipose tissue-derived stem cells, mounted in an adequate bioreactor for conditioning and maturation. Our tissue preparation methods encourage the attachment and retention of endothelial cells under simulated physiological conditions.
Collaborator: Dr. Chris Wright
3D cell-based structures for islets’ regeneration (Creative Inquiry)
Pancreatic islet transplantation is an efficient therapeutic strategy in type I diabetes, but often rejection and poor function of islets occur after transplantation. A new tissue engineering approach has been recently described in the literature, based on the developing of multicellular aggregates or spheroids from commercial pancreas-derived cell lines. In this project, the potential of mesenchymal stem cells to form cellular spheroids and differentiate into insulin-producing cells will be investigated. These structures can be used to study pathological mechanisms involved in diabetes, as well as the effect of different drugs used for the treatment of diabetes.
3D cell-based structures for myocardial regeneration (Creative Inquiry)
Mesenchymal stem cells have the potential to differentiate into cardiac cells, especially when grown as 3D spheroid structures. In this project, cell self-assembling techniques are considered in order to form stem cell spheroids able to generate cardiac-like microtissues. These structures can serve as in vitro models to: 1) study of normal and pathological processes that occur in cardiac tissues; 2) study the effect of drugs on cardiac cells (and mitigate the use of animal studies); 3) assemble as building blocks and generate larger-sized tissue constructs (a tissue engineering and regenerative medicine approach).






