Probing the Bioactivity, Conformation and Orientation of Adsorbed Proteins
The bioactive state of an adsorbed protein is substantially influenced by both the type of protein and the type of surface to which it is adsorbed. Furthermore, the bioactivity of a given protein that is adsorbed on a given surface can be substantially influenced by the orientation of the protein on the surface and adsorption-induced changes that might occur in the protein's structure.
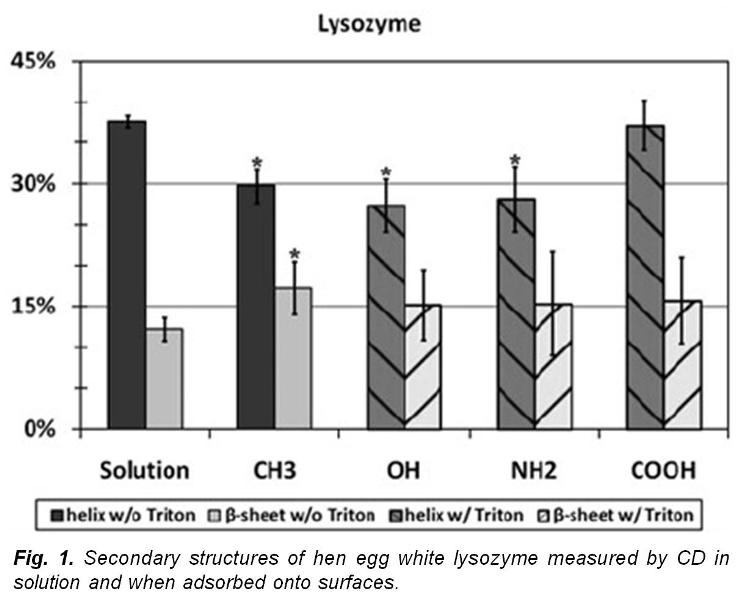 Circular Dichroism (CD) provides an extremely powerful and sensitive method to characterize the secondary structure of proteins (i.e., percent α-helix, β-sheet, and random loop content) both in solution and when adsorbed to surfaces. Our group developed CD methods to study the effect of adsorption on the secondary structure of proteins with high signal-to-noise ratio to provide a very high level of accuracy and reproducibility.
Circular Dichroism (CD) provides an extremely powerful and sensitive method to characterize the secondary structure of proteins (i.e., percent α-helix, β-sheet, and random loop content) both in solution and when adsorbed to surfaces. Our group developed CD methods to study the effect of adsorption on the secondary structure of proteins with high signal-to-noise ratio to provide a very high level of accuracy and reproducibility.
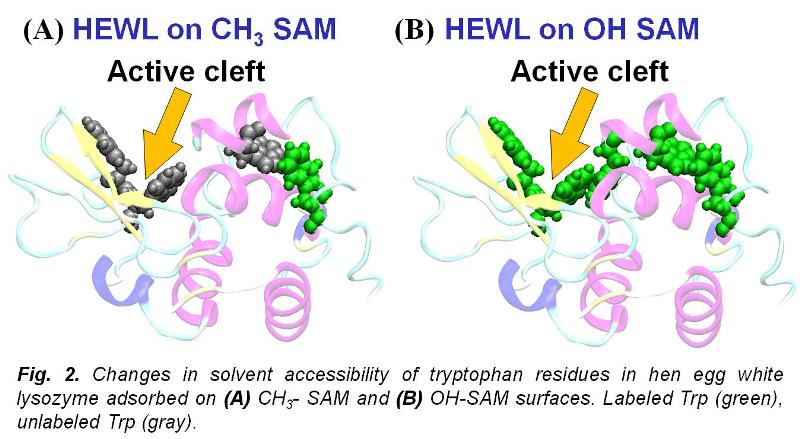
While CD provides an excellent means of measuring protein secondary structure, it provides very little information regarding the orientation and tertiary conformation of adsorbed proteins. To address this need we developed specific amino acid side-chain modification methods, which, when combined with mass spectrometry, provide this type of molecular-level information. In this method, a protein is first adsorbed to a surface and then it is chemically treated to label the solvent-accessible side chains of targeted amino acids. The protein is then digested from the surface and the positions of the labeled amino acids are identified by mass spectrometry. Since side-chain modification will only take place for amino acid residues that are solvent accessible, the change of the number and position of the modified amino acid residues before and after adsorption provides quantitative information regarding both the orientation and changes in the tertiary structure of the adsorbed protein. Combining the results from these analytical techniques, the bioactive state of any protein on any type of surface to which it is adsorbed can be assessed.
______________________________________
Defense Threat Reduction Agency (DTRA)
Grant number HDTRA1-10-1-0028 (PI: Latour)
