THE COTTLE GROUP
Principal Investigator: Dr. Renee Cottle
RESEARCH
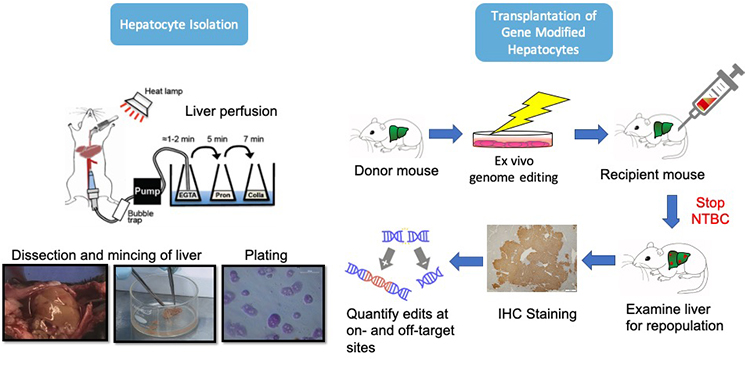
Inherited metabolic diseases (IMDs) are typically caused by autosomal recessive single gene mutations resulting in organ intoxication and if untreated, premature death. Advancements in newborn screening have made it possible to diagnose IMDs and commence treatment during infancy. Orthotopic liver transplantation is the only curative therapy available for severe IMDs of the liver, but it is limited by critical organ shortages and high risk of mortality from the procedure, transplant rejection and side effects from life-long immunosuppressive therapy. Although site-specific nucleases harnessed for gene editing in the human genome are rapidly advancing to clinical trials for IMDs of the liver, a major barrier for liver-directed gene editing-based therapies exists: The absence of safe and effective methods for delivering gene editing reagents into hepatocytes, the target cell in the liver. Viral delivery vectors, particularly adeno-associated viral vectors, are the most common delivery method for introducing CRISPR-Cas9 in vitro. Although the platform of choice, adeno-associated viral vectors are limited by risks of immunogenicity, off-target insertional mutagenesis, and genotoxicity from persistent Cas9 expression. The Cottle Group develops nonviral approaches to deliver CRISPR-Cas reagents into primary hepatocytes ex vivo through electroporation and lipid nanoparticles. Nonviral delivery methods have the potential to address the challenges of viral-mediated delivery as they enable introduction of gene editing reagents in transient and potent forms, such as mRNA and ribonucleoproteins. However, transient forms of gene editing tools introduced into cells in vivo continue to be associated with a risk of cell-mediated activation of the immune system. The Cottle Group investigates whether gene editing ex vivo is a safer approach than in vivo gene editing in hepatocytes. We assess the engraftment and repopulation potential of gene modified hepatocytes in the liver using the fumarylacetoacetate hydrolase (Fah)-deficient mouse model C57BL/6 Fah∆exon5. The Cottle Group has capabilities to isolate primary hepatocytes from the liver and transplant them into C57BL/6 Fah∆exon5 mice. In addition, our research team is investigating the application of ex vivo gene editing methods in hepatocytes to develop in vitro models of inherited metabolic diseases for drug screening and liver biology studies.