THE COTTLE GROUP
Principal Investigator: Dr. Renee Cottle
RESEARCH
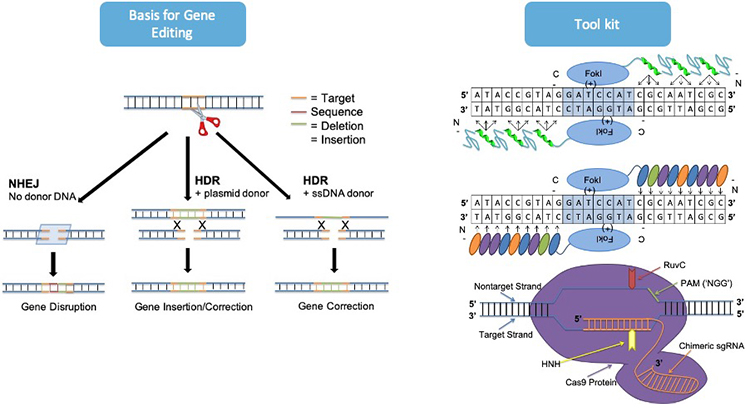
Gene editing technologies have unprecedented potential to revolutionize medicine: To cure, treat, or even prevent a wide array of genetic and acquired diseases. The objective of therapeutics that rely on gene editing technologies is to precisely replace a native DNA sequence with a desired modified sequence within target cells to correct a disease-causing mutation, disrupt gene expression, make small or large deletions of nucleotides to restore the gene reading frame, or insert a new gene sequence. . The basis for gene editing using site-specific nucleases is activation of a double-strand break at precise locations in the genome. Double strand breaks are repaired by endogenous DNA repair pathways: nonhomologous end joining (NHEJ) and homology-directed repair (HDR). The NHEJ pathway can introduce a small insertion or deletion at the break site that can cause frameshifts or exon skipping mutations. In contrast, HDR results in high fidelity repairs using a donor template DNA. The co-delivery of donor template DNA and site-specific nucleases causes incorporation of nucleotides from the donor DNA into the genome. Site-specific nucleases are divided into two broad categories: protein-based tools, consisting of zinc-finger nucleases and transcriptional activator-like effector nucleases, and RNA-guided nucleases, consisting of CRISPR-Cas nucleases and nickases. The Cottle Group specializes in designing RNA-guided gene editing tools, including the type II CRISPR-Cas system of Streptococcus pyogenes that consists of a Cas9 nuclease and guide RNA, to target therapeutic genes associated with inherited metabolic diseases affecting the liver. In addition, the group investigates design rules for making synthetic donor templates to make precise edits through the HDR pathway. One major disadvantage of CRISPR-Cas nucleases is their high levels of gene editing at unintended sites, which have potentially deleterious effects, including chromosomal deletions and translocations. Therefore, careful design of the guide RNA is critical to its successful application. The Cottle Group has capabilities to analyze the off-target activity of CRISPR-Cas nucleases using in silico tools to predict potential off-target sites and validate the nuclease specificity using targeted next generation sequencing on an Illumina Miseq system.