NANOBIOTECHNOLOGY LAB

Peptide nanocarriers for targeted delivery of RNAi therapeutics
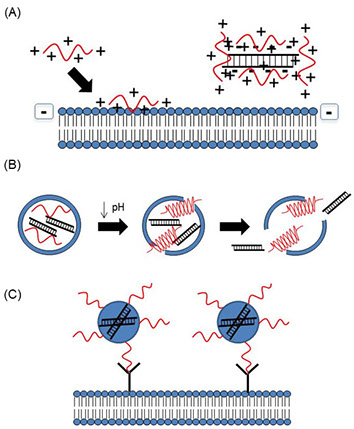
Although RNAi therapy is promising, siRNAs delivered in vivo face significant barriers including serum instability, renal excretion, and rapid uptake and clearance by the reticuloendothelial system (RES) [1,2]. Additionally, due to their large molecular weight and negative charge, naked siRNAs are unable to diffuse across the cellular membrane [3]. Many strategies using nanotechnology for siRNA delivery have been investigated to address these barriers, such as several classes of peptides. These peptides include cell penetrating peptides (CPPs), pH-sensitive membrane active peptides (PMAPs), and homing peptides [4-6], which mediate cellular uptake, membrane fusion/endosomal escape, and targeted delivery, respectively (Fig. 1). Although each class of peptides has been used individually to deliver siRNAs [7,8], few studies have combined different classes of peptides to overcome multiple barriers Therefore, our lab is developing tandem peptides containing several classes of peptide sequences and determining whether these peptides improve targeted delivery and uptake of bioactive siRNAs into tumor tissue
Tailorable drug delivery systems for combination therapy
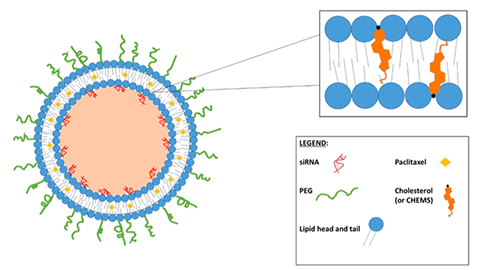
Advances in nanotechnology have led to the development of multifunctional nanoparticles that allow the delivery of multiple drugs, nucleic acids, targeting moieties, and imaging agents to diseased tissue. Due to heterogeneity of tumor tissues and drug resistance mechanisms, many cancer patients may benefit from treatments combining multiple therapeutics [9]. Combination therapies which include multiple drugs and gene therapy have been proven to increase drug sensitivity and therapeutic efficacy, particularly in cancer treatment [10,11]. Liposomal nanoparticles are ideal for combination therapy due to the ability to tailor their properties including size, surface charge and hydrophobicity. Alternating hydrophilic and lipophilic regions within the lipid bilayers of liposomes allow loading of both hydrophilic and hydrophobic drugs. Nucleic acids can also be encapsulated in various types of charged liposomes for gene therapy. For instance, stable nucleic acid lipid particles (SNALPs) are liposomes that have neutral outer surfaces and allow nucleic acid loading through electrostatic interaction with cationic lipids in the interior [12]. Lipoplexes offer similar capabilities in which nucleic acids may be embedded between cationic lipid bilayers of the multilamellar liposomes [12]. Additionally, multifunctionality can be created by conjugating targeting moieties and incorporating imaging agents to enable cell/tissue specific uptake and image-guided therapy, respectively [13,14]. Therefore, our lab is developing multifunctional SNALPs and lipoplexes that enable tailorable combination therapy for the treatment of various cancers.
Implantable scaffolds for controlled release of therapeutics
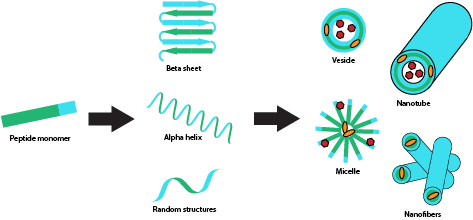
Therapeutic treatment of many disease models, including cancer, requires multiple dosing of therapeutic agents, which often results in harmful side effects such as myelosuppression and cardio- and neurotoxicity due to repeated systemic exposure [9,15,16]. Furthermore, disease models that result in tissue injury, often require scaffolding materials to support tissue regeneration [17,18]. The development of implantable extended drug release systems is necessary to enable sustained local therapeutic delivery, circumventing multiple dosing of drugs while also minimizing exposure to normal tissue and cells. Additionally, scaffolds provide matrix support for cellular implantation and growth for tissue regeneration applications. Hydrogels have been extensively investigated as implantable scaffolds for drug delivery due to their unique properties. Hydrogels are water-soluble polymers that form porous three-dimensional cross-linked networks which enable drug loading [19]. Hydrogels are generally biocompatible and biodegradable, making them good candidates for in vivo application. Their degradation can be engineered to be triggered by pH, temperature, or enzymatic activity, allowing for controlled release based on environmental conditions [20]. Additionally, peptides can be designed to self-assemble into hydrogels, by designing sequences of polar and non-polar tandem repeats and have shown promise in tissue engineering applications. Therefore, our lab is developing self-assembling polymeric and peptide scaffolds for controlled release of multiple therapeutics, including drugs, siRNAs, pDNAs, growth factors, and proteins for tissue engineering following injury and cancer therapy (Fig. 3).
References
[1] Gavrilov K, Saltzman WM. Therapeutic siRNA: Principles, Challenges, and Strategies. The Yale Journal of Biology and Medicine. 2012; 85(2):187-200.
[2] Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009; 8(2):129-38.
[3] Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010; 12(4):492-503.
[4] Li W, Nicol F, Szoka FC, Jr. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Advanced drug delivery reviews. 2004; 56(7):967-85.
[5] Mo RH, Zaro JL, Shen W-C. Comparison of Cationic and Amphipathic Cell Penetrating Peptides for siRNA Delivery and Efficacy. Molecular Pharmaceutics. 2012; 9(2):299-309.
[6] Turner JJ, Arzumanov AA, Gait MJ. Synthesis, cellular uptake and HIV-1 Tat-dependent trans-activation inhibition activity of oligonucleotide analogues disulphide-conjugated to cell-penetrating peptides. Nucleic acids research. 2005; 33(1):27-42.
[7] Oliveira S, van Rooy I, Kranenburg O, Storm G, Schiffelers RM. Fusogenic peptides enhance endosomal escape improving siRNA-induced silencing of oncogenes. International Journal of Pharmaceutics. 2007; 331(2):211-4.
[8] Boisguerin P, Deshayes S, Gait MJ, O'Donovan L, Godfrey C, Betts CA, et al. Delivery of therapeutic oligonucleotides with cell penetrating peptides. Advanced drug delivery reviews. 2015.
[9] Komarova NL, Boland CR. Cancer: Calculated treatment. Nature. 2013; 499(7458):291-2.
[10] Lopez JS, Banerji U. Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nature reviews Clinical oncology. 2016.
[11] Tsouris V, Joo MK, Kim SH, Kwon IC, Won YY. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnology advances. 2014; 32(5):1037-50.
[12] Xia Y, Tian J, Chen X. Effect of surface properties on liposomal siRNA delivery. Biomaterials. 2016; 79:56-68.
[13] Kono K, Takashima M, Yuba E, Harada A, Hiramatsu Y, Kitagawa H, et al. Multifunctional liposomes having target specificity, temperature-triggered release, and near-infrared fluorescence imaging for tumor-specific chemotherapy. Journal of Controlled Release. 2015; 216:69-77.
[14] Xiang B, Dong D-W, Shi N-Q, Gao W, Yang Z-Z, Cui Y, et al. PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer. Biomaterials. 2013; 34(28):6976-91.
[15] Henri C, Heinonen T, Tardif JC. The Role of Biomarkers in Decreasing Risk of Cardiac Toxicity after Cancer Therapy. Biomarkers in cancer. 2016; 8(Suppl 2):39-45.
[16] Hoeffner EG. Central Nervous System Complications of Oncologic Therapy. Hematology/oncology clinics of North America. 2016; 30(4):899-920.
[17] Roura S, Galvez-Monton C, Bayes-Genis A. Fibrin, the preferred scaffold for cell transplantation after myocardial infarction? An old molecule with a new life. Journal of tissue engineering and regenerative medicine. 2016.
[18] Tian L, Prabhakaran MP, Ramakrishna S. Strategies for regeneration of components of nervous system: scaffolds, cells and biomolecules. Regenerative biomaterials. 2015; 2(1):31-45.
[19] Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008; 49(8):1993-2007.
[20] McKenzie M, Betts D, Suh A, Bui K, Kim LD, Cho H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules (Basel, Switzerland). 2015; 20(11):20397-408.
ANGELA ALEXANDER-BRYANT
Assistant Professor
Director of Diversity and Inclusion
Department of Bioengineering
Clemson University
Office: 501-3 Rhodes Research Center
Clemson University
Clemson, SC 29634
Email: AngelaA@clemson.edu
Phone: 864-656-5232
