Research
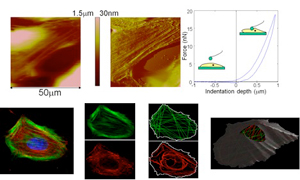
Single cell structure-function relationship
Cells, particularly cardiovascular cells, can undergo significant cytoskeletal remodeling in response to various stimuli. This remodeling leads to changes in the cell mechanical properties that may eventually contribute to a variety of physiological or pathological conditions. We are currently conducting several studies aimed at understanding the relation between cardiovascular cell structure and cell mechanical properties. We are investigating how cell-cell and cell-matrix interactions modulate cardiac cell mechanical response, how cell mechanical properties vary during cell differentiation, and how to predict cell mechanical properties from optical imaging data.
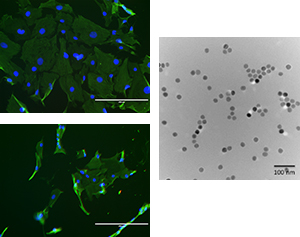
Nanoparticles and cell function
Nanoscience has shown great potential to elucidate a wide variety of biomedical problems. In addition, nanoparticles are currently being developed for many specific applications from novel cancer therapies to improved tennis racquet design. However, the long-term effects of these new nanomaterials are not well understood. Our goal is to understand how nanoparticles interact with individual cells and how these interactions may alter the cell function. We are currently studying the effects of heparin-coated magnetic nanoparticles on the proliferation of vascular smooth muscle cells and endothelial cells. In addition, we would like to design a delivery method that optimizes the drug uptake and efficiency to treat neointimal hyperplasia and prevent restenosis.
Integration of Dental Pulp Stem Cells (DPSCs) on a Spider Silk Matrix
The overall health of a tooth is heavily influenced by the dental pulp in the root, which contains not only contains the nerves and blood but also undifferentiated pulp cells (PDSCs). These cells are similar to bone marrow cells and, under proper conditions, they can differentiate to replace damaged odontoblasts. The goal of this project is to understand what factors influence dental pulp cell differentiation. For instance, dentin and enamel have very specific nanoscale and microscale architecture. We are currently investigating the effect of substrate topography and structure on the differentiation and function of dental pulp cells.
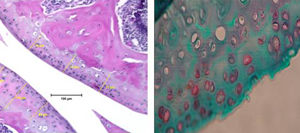
Radiation and cartilage tissue
In addition to microgravity, astronauts are also exposed to complex radiation while in space. This radiation, caused by solar events, may compound microgravity’s effects on articular cartilage. Additionally, many cancer patients undergo radiotherapy as a standard treatment, putting them at risk of damaging or altering the mechanical properties of their cartilage. Therefore, the goal of this project is to understand how radiation affects articular cartilage. We are using atomic force microscopy (AFM) to test the mechanical properties cartilage from mice that have been irradiated with simulated cosmic rays. Mice tissues are very small and therefore it is very difficult to characterize their material properties using traditional testing techniques.
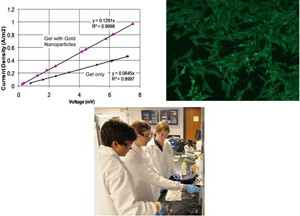
Nanoparticle-modified hydrogels for improved electrical conductive properties
Electrically conductive biocompatible gels are useful as coatings on electrode implants interfaces as well as for various engineered tissue constructs. Currently, many groups are investigating the development of new electrically conductive polymers for these applications. Unfortunately, one limitation of these new polymers is that they are not yet approved by regulatory agencies and some of these materials have limited biocompatibility. When an electrode is implanted, a collagenous fibrous layer tends to form around it due to immune responses and poor biocompatibility. This collagen layer allows for good cell attachment but creates large resistance for electrical signaling to the electrode surface. In this study, we propose to use nanoparticles to vary the conductance of collagen. These new composite gels are still biocompatible but have improved electrical properties compared to unmodified gels.
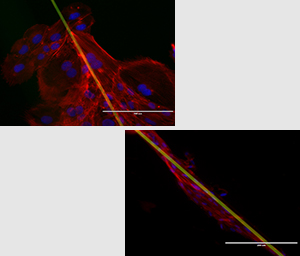
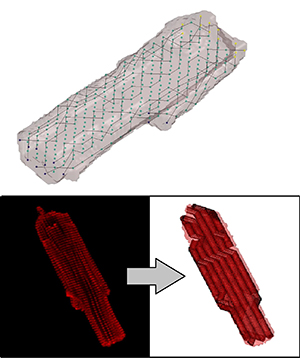
Cardiac Electromechanical Modeling
Following myocardial infarction, damaged cardiac tissue undergoes remodeling in an attempt to repair and strengthen the heart. As a part of this remodeling process, myocytes change size and shape, and there is an infiltration of fibroblasts into the myocardial space. This drastic change in the tissue structure leads to changes in the electrical and mechanical function, the extent of which are not fully understood. Because of the difficultly of studying this environment in vivo or adequately recreating it in tissue culture, the long term goal of this project is to develop an image based computational platform to model the mechanical and electrical behavior of this, and other unique cardiac environments. Based mainly on confocal microscopy imaging, we employ a variety of image processing and computational methods to recreate cellular and tissue geometries for use in Finite Element Modeling (FEM).