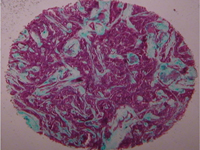
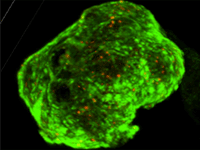
Vascular stents are a means of increasing blood vessel patency with a small, often metallic, mesh tubes implanted endovascularly. While metallic alloys such as nitinol or stainless steel are often chosen for their superior mechanical properties, stents still fail at rates as high as 12% due to deployment complications, stent geometry, material properties, and surface coatings. Carbon-based coatings have been previously used to improve implant properties, however many are still limited in their efficacy as a functional stent material. Nitinol substrates coated with an atomically smooth graphene layer are expected to improve physiological response by 1) reducing protein adsorption on the surface. 2) Reducing the relative amount of fibrinogen adsorption, a protein involved in thrombus formation, compared to albumin, a serum protein. 3) Decreasing charge transfer from adsorbed proteins to the substrate, a physical process indicating protein conformational change. 4) Improving cell viability and morphology on the substrate.