Integrating Ice-free Vitrification and Nanowarming for Banking of Large Osteochondral and Meniscus Grafts [Funded by NIH SBIR (Fast Track) and Musculoskeletal Transplant Foundation (MTF)]
Due to the complexity of the structure-function relationship in the osteochondral interface and meniscus, a full functional recovery of the damaged tissue is beyond the regeneration capacity of the human body. Cryopreserved allograft transplantation is a common procedure that attempts to restore the biomechanical functions of the osteochondral interface and meniscus. However, lack of donor-cell viability and tissue lacunae due to ice crystal formation are two major barriers for cryopreservation and affect allograft survival and transplant outcomes in clinical practice.
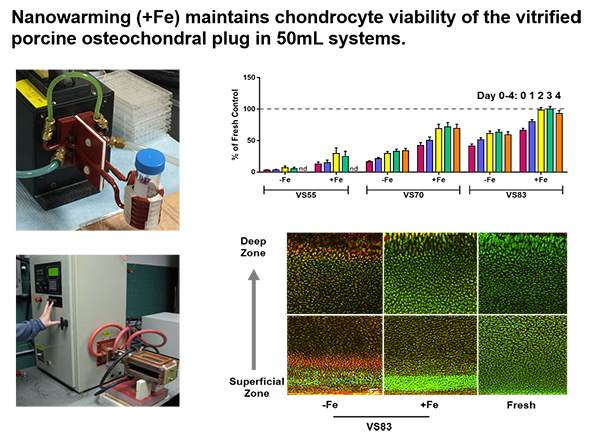
Our hypothesis is that complex tissues (e.g., large osteochondral, meniscus) can be cryopreserved and rapidly rewarmed using ice-free cryopreservation and radio frequency-induced warming of nanoparticles without devitrification associated ice damage or cryoprotectant induced toxicity.
The objective of this project is to characterize nanowarming of ice-free vitrified femoral osteochondral condyle and meniscus for the purpose of long-term viable allograft storage, by assessing cell viability and extracellular matrix quality (i.e., structure-function relationship). Success of this novel technology will result in effective cryopreservation methods for tissues and organs where banking is currently not feasible due to ice damage employing conventional cryopreservation freezing methods. This would permit better donor-recipient matching with improved post-transplant outcomes.