Research
The Drug Design, Development, and Delivery (4D) Lab’s mission is to alleviate pathological conditions and promote functional tissue regeneration through biomaterial-based synthesis, formulation, and delivery of therapeutic compounds. Our research focuses on the understanding of drug and gene delivery in biological systems and developing new therapeutics and biomaterials for the diagnosis and treatment of many diseases. We are currently working in the following areas:
Drug-eluting Medical Devices
1. Drug-loaded Electrospun Fiber for Surgical Suture
Free tissue transfer is a widely used technique for soft tissue reconstruction in which surgical sutures are used to connect the graft and implant site vasculature. One of the most common complications that can lead to graft failure is anastomotic thrombosis. Heparin is the most commonly used anti-coagulant to prevent anastomotic thrombosis. Electrospinning technique is a simple and versatile method for developing polymeric fibers with diameters ranging from submicrons to several nanometers. In addition, electrospun nanofibers provide higher surface area available for modifications with different functional groups than conventional melt-spun fibers. The objective of this study is to develop heparin-immobilized electrospun nanofibers as a microvascular suture. We hypothesize the positively charged electrospun fiber may interact with negatively charged heparin via electrostatic interactions and provide sustained and controlled release of heparin from the suture (Scheme 1). Positively charged nanofiber sutures were successfully fabricated by the electrospinning technique. Currently, we are preparing electrospun nanofibers with various compositions to optimize heparin loading efficiency, and evaluating the anti-thrombin activity of heparinized electrospun fibers as surgical sutures for vascular anastomosis.
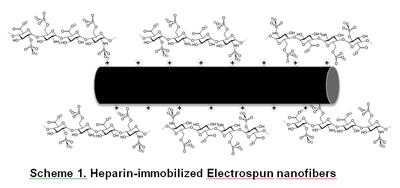
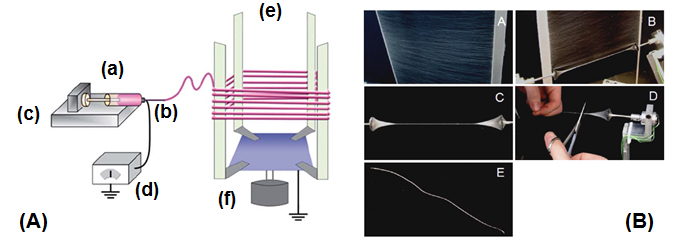
Fig 1A. Schematic diagram of the electrospinning apparatus for collecting nanofibers. (a) polymer solution (b) needle (c) syringe pump (d) high-voltage power supply (e) rotating collector (f) motor. Fig 1B. A high voltage power supply was connected to the syringe through a stainless-steel needle and the nanofibers were collected on the custom-made collecting and twisting devices..
Collaborators:
Dr. Konstantin Kornev (School of Materials Science & Engineering, Clemson University)
Dr. Robert Brown (Head & Neck Surgeon, Greenville Health System)
2. Drug-loaded Tissue Adhesives
Several different techniques have been used for traumatic wound treatment such as sutures, tapes, staples, ligating clips, and adhesives. Octylcyanoacrylate received FDA approval in 1998 and is marketed for external wound closure after lacerations or incisions. Tissue adhesives have widespread appeal due to their ease and speed of application. Internal application of cyanoacrylates has been limited by slow degradation rate of existing products for topical use and the toxicity of presently available compositions with faster degradation. The objective of this project is to develop a cyanoacrylate-based tissue adhesive product for internal wound closure that is biodegradable and provides controlled-release of therapeutic compounds. Our approach is based on: 1) the incorporation of low molecular weight polyethylene glycol (PEG) in cyanoacrylates that will increase hydrophilicity, accelerate water uptake, and increase degradation rate and 2) drug encapsulation within poly (cyanoacrylate) nanoparticles that can be homogeneously and stably mixed with cyanoacrylate monomers and released from polymerized adhesives. In vivo biocompatible study were performed. Small surgical incisions (0.2 cm deep x 1 cm long) were made in both the biceps femoris hind limb muscles of adult Sprague-Dawley rats (n=4 / treatment group). Figure 1 shows in vivo biocompatible study of various cyanoacrylate formulations. Both macroscopic and histological observation demonstrated visible degradation of OCA / MCA and MCA-based formulations between 7 days and 4 weeks, while OCA formulations were relatively unchanged. Figure 2 shows poly(butylcyanoacrylate) (PBCA, Adhezion Biomedicals, Inc) particles with PMX (Antibiotics) prepared by anionic emulsion polymerization with scanning electron microscopy (SEM). Antibacterial activity of PMX-loaded PBCA nanoparticles was measured indirectly by the agar diffusion method and PMX-loaded PBCA particles showed similar antibacterial activity with free PMX, where drug-unloaded PBCA particles showed no antibacterial activity (Figure 3).
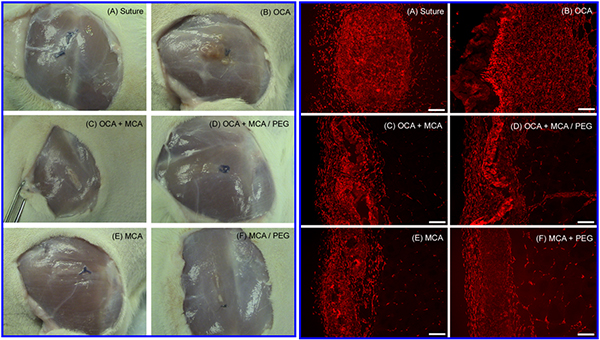
Fig 1.Left : Macroscopic histological appearance at 4 weeks of suture controls (A) and various cyanoacrylate adhesives formulations with and without PEG (B-F), and Right: Immunohistochemical analysis of fibroblast accumulation at 4 weeks in response to suture control (A) and various cyanoacrylate formulations with and without PEG (B-F).
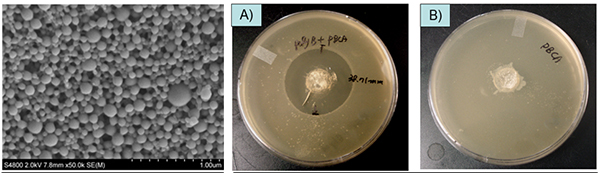
Fig 2. SEM images of PMX-loaded PBCA nanoparticles (left)
Fig 3. Antibacterial activity of PMX-loaded PBCA nanoparticles by the agar diffusion method (Center, Right)
Collaborators: Dr. Ken Webb (Bioengineering, Clemson University)
Funding: Adhezion Inc.



