Research
The Drug Design, Development, and Delivery (4D) Lab’s mission is to alleviate pathological conditions and promote functional tissue regeneration through biomaterial-based synthesis, formulation, and delivery of therapeutic compounds. Our research focuses on the understanding of drug and gene delivery in biological systems and developing new therapeutics and biomaterials for the diagnosis and treatment of many diseases. We are currently working in the following areas:
Target-specific Polymeric Prodrug System
Site-specific delivery is a useful strategy to improve the therapeutic properties of a drug. Controlling drug distribution in this manner enables the attainment of therapeutic drug concentration at the target site, while minimizing systemic levels and associated side effects. Colon-specific drug delivery involves transport of a drug to the large intestine,which is the disease site. The major advantages of colon-specific delivery of orally administered drugs are 1) more efficient treatment of colonic diseases such as Crohn’s disease, ulcerative colitis, colon and rectal cancer, and amoebiasis can be achieved by avoiding systemic absorption and reducing undesirable side effects and 2) the absorption of many protein and peptide drugs, which are unstable in the upper gastrointestinal tract (GIT), may be more beneficial effective through the large intestine due to the lack of digestive enzymes.
Colon-specific bi-functional polymeric prodrug for treatment of amebiasis
The amoebic parasite
Entamoeba histolytica is the causative agent of hemorrhagic enteric colitis and liver abscess. Once ingested, the trophozoites accumulate in the large intestine and may penetrate the mucus layer and adhere to the underlying epithelial cells in the colonic lumen. This adhesion involves the amoebic surface protein referred to as the galactose/N-acetyl galactosamine lectin and the adhesion can be effectively blocked in vitro by the addition of galactose (Gal) and N-acetyl galactosamine. Metronidazole (Mz) is one of most effective drugs for amebiasis, but it is likely to be more effective against systemic amebiasis than intestinal amebiasis. Therefore, colon-specific delivery of metronidazole and galactose is desirable for the efficient treatment of amebiasis developed at colonic sites. The objective of this study is to develop bi-functional polymeric amebicidal therapeutics, Galactose-Dextran-Metronidazole (Gal-Dex-MM) prodrugs as colon-specific therapeutics to efficiently treat E. histolytica infection through two mechanisms:1) delivery of metronidazole, to kill the amoebae, and 2) delivery of galactose that may inhibit adhesion of parasite to host cells. We hypothesized that Gal-Dex-MM prodrugs can stably pass through the upper GIT and that drug release will only occur after degradation of the dextran polymer backbone by endodextranase enzymes that are derived from microbes residing in the colon (Scheme 1).
We found that Dex-MM and Gal-Dex-MM are chemically and enzymatically stable in the presence of acidic pH and/or esterase enzymes as well as tissue contents derived from the upper GIT and that metronidazole release only occurs in the presence of dextranase or tissue contents derived from the colon. Metronidazole released from Gal-Dex-MM retained amoebicidal activity and released galactose efficiently blocked parasite-host cell interactions. Currently, we are evaluating the efficacy and cytotoxicity of Gal-Dex-MM in a mouse amoebic ulcer model
in vivo.
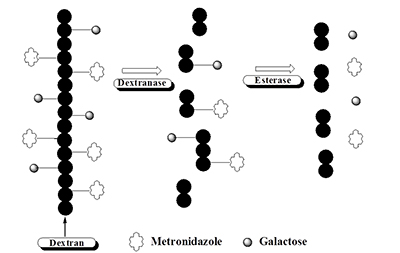
Scheme 1. Proposed release mechanism of metronidazole and galactose from Gal-Dextran-MM conjugate in the colon
Collaborator: Dr. Lesly Temesvari (Biological Science, Clemson University)
Funding: NIH RO3 (1R03AI076869) from NIAID (National Institutes of Allergy and Infectious Disease)
