The Gilbert Biomaterials and Regenerative Medicine Laboratory
Principal Investigator: Dr. Jeremy Gilbert
RESEARCH: CORROSION AND IMPEDANCE TESTING OF MEDICAL ALLOYS
Corrosion and Impedance Testing of Medical Alloys
Corrosion is a result of metal oxidation and reduction reactions where metal electrons begin to dissolve into the surrounding environment. The solution a metal is surrounded by, influences the rate of corrosion greatly. Biologically, inflammation, cell type and even tissue type play a role in corrosion of these passive metals. One way to measure the corrosion characteristics of metals and their environment is to measure their oxide’s impedance. Impedance measurements maintain a set voltage but oscillate its frequency. Depending on a surface reaction to the changing frequency of applied voltage, we can define a solution resistance and a passivation resistance and capacitive properties of the oxide.
Our lab has patented the technology to shorten this experiment from hours to minutes using a symmetry based approach using the Randles Circuit model.
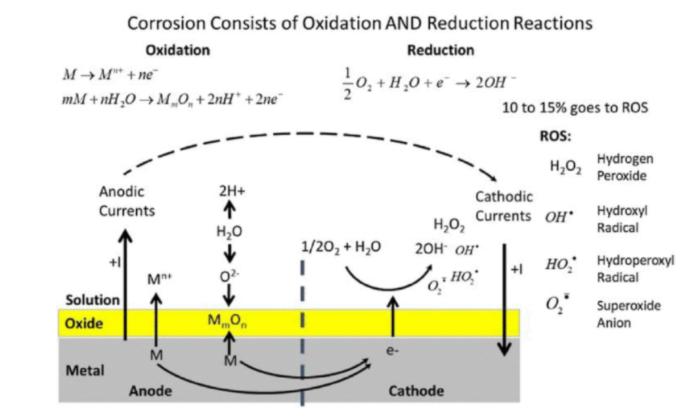
Figure: General summary of oxidation and reduction reactions that comprise corrosion of metallic biomaterials. Oxidation reactions, typically through the oxide film-covered surface, can form ions (in solution) or solid products like oxides or phosphates. Reduction reactions take oxygen and water in solution and make hydroxide ions and other reactive oxygen intermediates (e.g., reactive oxygen species [ROS]) Gilbert, J.L., Kubacki, G.W., 2015
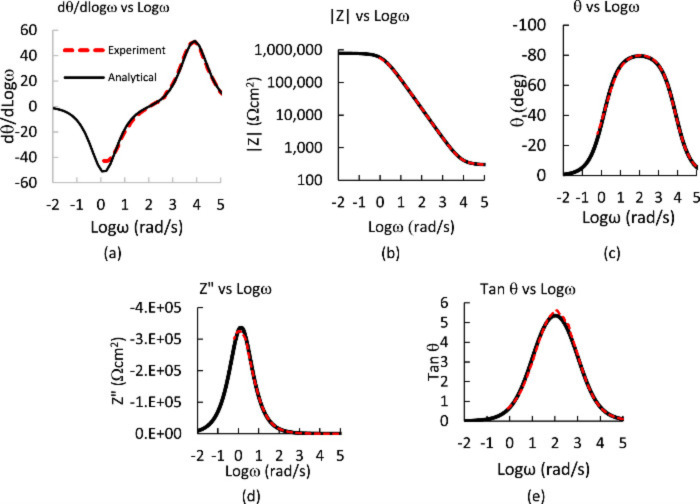
Figure: Randles circuit response (Rs = 100, Rp=106, C = 5e–5). (a) ∣Z∣ vs Logω, (b) θ vs Logω and dLog∣Z∣ vs Logω, and (c) dθ/dLogω (from Eqs. 20 and 21). Note the symmetry of the two peaks of opposite sign but similar amplitude and shape for the phase derivative (c). Each is positioned at a characteristic frequency where the Log∣Z∣ transitions from capacitive to resistive behavior and there is a cross over frequency at the peak on the θ vs Logω plot (b). Gilbert, JL., and Khulla Pr. J.Elec Soc (2020)
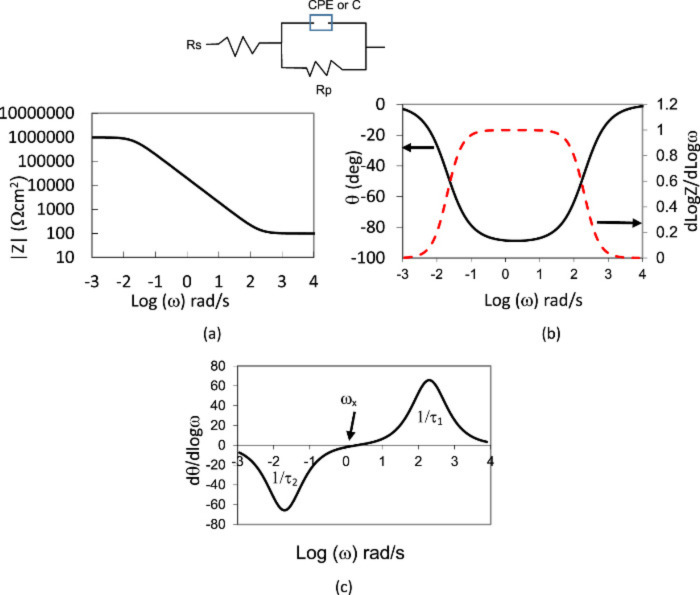
Figure: CoCrMo experimental EIS data (red) and CPE-Randles analytical equation (black) for: (a) phase derivative, (b) ∣Z∣, (c) θ, (d) Z'', and (e) Tanθ using the values obtained from the fitting approach. Gilbert, JL., and Khulla Pr. J.Elec Soc (2020)
Recent Publications
1. Kurtz, M. A., Wessinger, A. C., Mace, A., Moreno-Reyes, A., & Gilbert, J. L. (2022). Corrosion Resistance of Additively Manufactured Titanium Alloys in Physiological and Inflammatory-Simulating Environments: Ti-6al-4v Versus Ti-29nb-21zr. Available at SSRN 4308028.Cathodic activation and inflammatory species are critical to simulating in vivo Ti-6Al-4V selective dissolution
2. Mace, A., Khullar, P., Bouknight, C., & Gilbert, J. L. (2022). Corrosion properties of low carbon CoCrMo and additively manufactured CoCr alloys for dental applications. Dental Materials, 38(7), 1184-1193.Synthetic periprosthetic synovial fluid development for in vitro cell‐tribocorrosion testing using the Taguchi array approach
3. Gilbert, J. L., & Khullar, P. (2020). Analysis of electrochemical impedance spectra using phase angle symmetry across log frequency. Journal of The Electrochemical Society, 167(2), 021505.
4. Liu, Y., Zhu, D., Pierre, D., & Gilbert, J. L. (2019). Fretting initiated crevice corrosion of 316LVM stainless steel in physiological phosphate buffered saline: Potential and cycles to initiation. Acta biomaterialia, 97, 565-577.